Goniobasis or "Elimia" clavaeformis
> Habitat & Distribution
The range of P. clavaeformis (in its older, narrow sense) was given by Goodrich (1940) as "tributaries of the upper Tennessee River in Virginia, Tennessee and North Carolina; does not make the westward turn around Walden Ridge" (at Chattanooga). With our broader understanding of the taxon today we have documented P. clavaeformis populations (of the several subspecies) inhabiting tributaries of the Tennessee River extending westward well beyond Walden Ridge across the breadth of North Alabama, to include Elk River tributaries in Middle Tennessee. Pleurocerid populations indistinguishable from P. clavaeformis also inhabit the Alabama/Coosa drainage, and river systems further west as well.
Our modern surveys have also confirmed scattered P. clavaeformis clavaeformis populations inhabiting the Bluestone River and Wolf Creek, which drain toward The Ohio through the New/Kanawha system in western Virginia. We would subtract the state of North Carolina from the range of P. clavaeformis as suggested by Goodrich (1940), however, from which we have never seen any bona fide records.
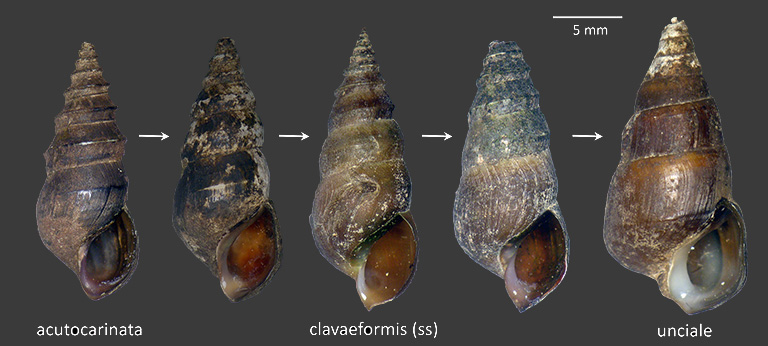
Populations of P. clavaeformis bearing shells of the typical (s.s.) form are most dense in creeks and streams of wadeable size. Populations inhabiting smaller streams of East Tennessee may bear strongly carinate shells referable to the subspecies acutocarinata (Lea 1841). Populations inhabiting the larger rivers tend to be more robustly-shelled, and in modern times have been referred to the subspecies unciale or uncialis (Haldeman 1841). More about both of these (related) phenomena below. The FWGNA incidence rank for all three subspecies considered together is I-5.
> Ecology & Life History
As the "pleurocerid in residence" at the Oak Ridge National Laboratory, Pleurocera clavaeformis has played a key role in quite a number of excellent studies of stream ecology (e.g., Steinman 1991, Hill 1992, Rosemond et al. 1993, Hill et al. 2010). Grazing by populations of P. clavaeformis can have a significant impact on algal biomass, primary productivity, and periphyton community structure. For a review see Dillon (2000: 86 - 91).
Like other pleurocerids, P. clavaeformis is dioecious, eggs being deposited on hard substrates from spring to mid-summer. Eggs are spirally arranged in masses of 2-15 or more, with a tough, membranous outer covering to which sand grains typically adhere (Smith 1980, Jokinen 1992). Although we are unaware of any study specifically directed toward the life history of P. clavaeformis, it seems reasonable to expect that two years will be required for maturity, and that several years of iteroparous reproduction can be expected thereafter, as is the case for pleurocerids generally (Dazo 1965). This is life cycle Hi of Dillon (2000: 156 - 162).
> Taxonomy & Systematics
Four populations of P. clavaeformis were included in the allozyme study of Dillon & Robinson (2007a). The species is quite distinct genetically. Our survey returned no evidence of hybridization with P. simplex, P. gabbiana, or P. troostiana, the other species of Pleurocera with which it often occurs, but see my essay of 20Aug25 from the link below for speculation in this regard.
The shells borne by populations of Pleurocera clavaeformis demonstrate great variety in shell carination, varying in overall length-to-width ratio and robustness as well. Populations inhabiting smaller streams are more slender and may bear a single carination (sometimes quite prominent) around mid-whorl. Such populations were described by Isaac Lea (1841) as Melania acutocarinata. The position of Lea's acutocarinata first in H & A Adams' (1858) alphabetical list of 12 random pleurocerid nomena resulted in its selection as the type of the Genus Elimia by Pilsbry & Rhoades (1896).
Populations in moderately-sized streams are carinate around the upper whorls only, showing the typical shell form which Tryon, Goodrich, and most 20th-century workers traditionally identifed as Goniobasis clavaeformis. Yet other populations, especially those inhabiting larger rivers, bear broader shells with carination shifted down toward the anterior periphery of the whorl. Such populations have historically been identified as Pleurocera unciale or uncialis (Haldeman 1841). The phenomenon of shifting genus-level taxonomy with increasing river size was first reported by Dillon & Robinson (2007b). See my essay of 20Feb07 [link below] for more.
In 2011 I extended the research of Dillon & Robinson (2007b) across east Tennessee to north Georgia, and in 2013 Dillon, Jacquemin & Pyron documented an identical phenomenon involving P. canaliculata populations further downstream. The results of those studies (Dillon 2011, Dillon et al. 2013) prompted a significant re-evaluation of higher taxonomic categories in the Pleuroceridae, carrying both the generic nomina Goniobasis and Elimia into synonymy under Pleurocera. The best entry into this long-running taxonomic controversy would be my essay of 23Mar11, entitled Goodbye Goniobasis, Farewell Elimia [link below].
It seems likely to us that the difference in shell robustness that led taxonomists to distinguish Elimia acutocarinata and Goniobasis (or Elimia) clavaeformis from Pleurocera unciale for almost 200 years may be ecophenotypic responses to differences in physical disturbance or predation pressures as small creeks grow into large rivers. This phenomenon was dubbed "cryptic phenotypic plasticity" by Dillon and colleagues (2013). See my essay of 3June13 from the link below for more.
We have suggested the retention of the nomena acutocarinata (Lea 1841) and unciale (Hald 1841) as subspecies, however, by virtue of their indexing function to the older literature. See my essays of 4Feb14, 5Mar14, and 20Aug25 for further elaboration.
The diploid number of P. clavaeformis (as either acutocarinata or as unciale) is 2n = 34 (Dillon 1991).
> Maps and Supplementary Resources
- The range of shell morphological variation in the Indian Creek (Va) population of Pleurocera (formerly Goniobasis) clavaeformis, from the report of Dillon & Robinson (2007b).
- Pretty photo of living P. clavaeformis clavaeformis, courtesy of Chris Lukhaup.

> Essays
- In my essay of 20Feb07 I coined the term "Goodrichian Taxon Shift" to describe the (apparently ecophenotypic) response of P. clavaeformis to increasing stream size.
- The phenomena documented in my 20Feb07 essay were extended to included pleurocerids ranging through east Tennessee into the Mobile Basin of Alabama in my essay of 12Oct09, "Pleurocera Puzzles."
- Taxonomic controversy has surrounded the generic nomina Pleurocera, Goniobasis, and Elimia for many years. In 23Mar11 I posted a third essay in this series, generalizing my research findings on this subject to bid, "Goodbye Goniobasis, Farewell Elimia." Links are available from that essay to older resources on the whole taxonomic mess.
- In my essay of 3June13 I documented an identical "Goodrichian taxon shift" in "Pleurocera acuta is Pleurocera canaliculata." This prompted my colleagues and me to broaden the concept and rechristen it "cryptic phenotypic plasticity."
- Pleurocera claveformis clavaeformis received passing mention in my essay of 4Feb14, What Is a Subspecies?
- The tortuous taxonomic history of the nomen "unciale" served as a primary example in my follow-up essay of 5Mar14, What Subspecies Are Not.
- I reviewed the entire phenomenon of cryptic phenotypic plasticity in P. clavaeformis in my blog post of 2June16, The Shape-shifting Pleurocera of North Alabama. That essay featured a good figure illustrating the range of shell morphology displayed by a clavaeformis population sampled from the Paint Rock River.
- I formally recognized acutocarinata as a subspecies of P. clavaeformis in my blog post of 20Aug25, The birth, death, and resurrection of Melania acutocarinata. That post included a reproduction of Isaac Lea's original (1843) figure.
- A misunderstanding of the subspecific relationships between populations of P. clavaeformis bearing shells of the acutocarinata form upstream and the unciale form downstream contributed to the apparent polyphyly in the North American Pleuroceridae uncovered by Whelan et al. (2022). For more, see my essay of 14Oct25, Anchored hybrid enrichment and the prodigious clavaeformis confusion.
> References
Adams, Henry and Arthur (1854 - 1858) The Genera of Recent Mollusca Arranged According to Their Organization. In Three Volumes. London: J. Van Voorst. The Melanidae is covered in Volume 1, pp 293 311.
Dazo, B. C. 1965. The morphology and natural history of Pleurocera acuta and Goniobasis livescens (Gastropoda: Cerithiacea: Pleuroceridae). Malacologia 3: 1 - 80.
Dillon, R. T., Jr. (1989) Karyotypic evolution in pleurocerid snails: I. Genomic DNA estimated by flow cytometry. Malacologia, 31: 197-203.
Dillon, R. T., Jr. (1991) Karyotypic evolution in pleurocerid snails: II. Pleurocera, Goniobasis, and Juga. Malacologia 33: 339-344.
Dillon, R. T., Jr. (2000) The Ecology of Freshwater Molluscs. Cambridge, Cambridge University Press. 509 pp.
Dillon, R. T., Jr. (2011) Robust shell phenotype is a local response to stream size in the genus Pleurocera (Rafinesque, 1818). Malacologia 53: 265-277. [pdf]
Dillon, R. T. Jr., & K. B. Davis (1991) The diatoms ingested by freshwater snails: temporal, spatial, and interspecific variation. Hydrobiologia 210: 233-242.
Dillon, R. T. Jr., S. J. Jacquemin & M. Pyron (2013) Cryptic phenotypic plasticity in populations of the freshwater prosobranch snail, Pleurocera canaliculata. Hydrobiologia 709: 117-127. [html] [pdf]
Dillon, R. T., Jr., & J. D. Robinson (2007a) The Goniobasis ("Elimia") of southwest Virginia, I. Population genetic survey. Report to the Virginia Division of Game & Inland Fisheries, 25 pp. [PDF]
Dillon, R. T., Jr., & J. D. Robinson (2007b) The Goniobasis ("Elimia") of southwest Virginia, II. Shell morphological variation in Goniobasis clavaeformis. Report to the Virginia Division of Game & Inland Fisheries, 12 pp. [PDF]
Goodrich, C. (1913) Spring collecting in southwest Virginia. Nautilus 27: 81-82, 91-95.
Goodrich, C. (1940) The Pleuroceridae of the Ohio River drainage system. Occas. Pprs. Mus. Zool. Univ. Mich., 417: 1-21.
Haldeman, S.S. 1841. New species of shells, published October 5, 1841, by S. S. Haldeman. In: A monograph of the freshwater univalve Mollusca of the United States. J Dobson, Philadelphia.
Hill, W. R. (1992) Food limitation and interspecific competition in snail-dominated streams. Can. J. Fish. Aquat. Sci. 49: 1257-1267.
Hill, W. R., J. G. Smith & A. J. Stewart (2010) Light, nutrients, and herbivore growth in oligotrophic streams. Ecology 91: 518-527.
Jokinen, E.H. 1992. The Freshwater Snails (Mollusca: Gastropoda) of New York State. NY State Mus Bull 482, Albany, New York.
Lea, Isaac 1841. Continuation of Mr. Lea's paper on New Fresh Water and Land Shells. Proceedings of the American Philosophical Society 2: 11 15.
Lea, Isaac 1843. Description of New Fresh Water and Land Shells. Transactions of the American Philosophical Society (New Series) 8: 163 250.
Pilsbry, H. and S. Rhoads 1896. Contributions to the Zoology of Tennessee, Number 4, Mollusca. Proc. Acad. Nat. Sci. Phila. 1896:487-506.
Rosemond, A. D., P. J. Mulholland & J. W. Elwood (1993) Top-down and bottom-up control of stream periphyton: Effects of nutirents and herbivores. Ecology 74: 1264 - 1280.
Smith, D.G. 1980. Goniobasis virginica Gastropoda Pleuroceridae in the Connecticut River USA. Nautilus 94:50-54.
Steinman, A. D. (1991) Effects of herbivore size and hunger level on periphyton communities. J. Phycol. 27: 54 - 59.
Stewart, T. W., & R. T. Dillon, Jr. (2004) Species composition and geographic distribution of Virginia's freshwater gastropod fauna: A review using historical records. Am. Malac. Bull. 19: 79-91.
Whelan, N. V., Johnson, P. D., Garner, J. T., Garrison, N. L., & Strong, E. E. (2022) Prodigious polyphyly in Pleuroceridae (Gastropoda: Cerithioidea). Bulletin of the Society of Systematic Biologists, 1(2). https://doi.org/10.18061/bssb.v1i2.8419








