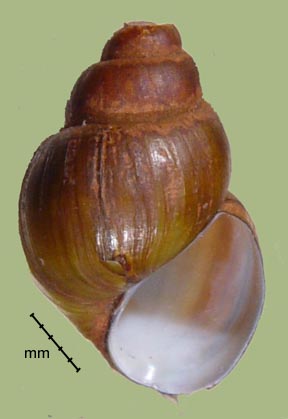
Campeloma "integra," limum, geniculum, parthenum, etc.
> Habitat & Distribution
Combined with all its probable synonyms, Campeloma decisum ranges throughout eastern North America, well up into Canada and down into Florida (Clarke 1981, Thompson 1999). Populations are common throughout most of our study area, in Atlantic drainages from Pennsylvania through Georgia into the Florida panhandle, and across all drainages of The Ohio and The Tennessee/Cumberland. They reach the limit of their range in eastern Nebraska and South Dakota, apparently excluded from the dryer (unglaciated) Great Plains further west.
Populations of Campeloma seem to reach maximum abundance burrowing in sandy bottoms where the current is sufficient to oxygenate the water column, especially in the larger rivers of the Interior Plains, the Piedmont, and the upper Atlantic Coastal Plain. They are not commonly found in small rocky streams, nor in lentic environments where summer temperatures might become extreme. FWGNA incidence rank I-5.
> Ecology & Life History
Little is known regarding the diet of Campeloma. The burrowing habit and peculiar radular morphology displayed by these snails imply an ability to filter feed, as has been documented for Viviparus. But the snails have almost certainly retained the ability to graze or harvest deposits on soft sediments as well. There are reports that Campeloma can be baited with carrion.
Parthenogenesis has evolved three times in the freshwater Gastropoda, all three occasions (Campeloma, Potamopyrgus, and the thiarids) in ovoviviparous brooders (Dillon 2000:109). Some populations of Campeloma appear comprised entirely of parthenogenic females, others appear to reproduce entirely by outcrossing, and some populations display a mixture of the two modes (Johnson & Bragg 1999, Johnson & Leefe 1999, Johnson 2000, Crummett & Wayne 2009). Crummett et al. (2013) reported evidence that parthenogenetic and sexual populations of Campeloma may differ in habitat.
Only a single year is required for maturation in some populations, two in others, and both semelparous and iteroparous reproduction have been reported (Vail 1978, Brown et al. 1989, Brown & Richardson 1992). Jokinen s (1983) analysis of the distribution of C. decisum in Connecticut and New York led her to classify it as an A-B tramp, typically present only in the more species-rich communities. Dillon s (2000: 360 - 363) USR reanalysis of these data suggested that C. decisum populations in Connecticut seem to be Undifferentiated with respect to life history adaptation.
> Taxonomy & Systematics
Clench (1962) reviewed all 49 specific nomina historically assigned to the genus Campeloma, reducing their number to 14 by synonymy. Burch (1989) further reduced the number of species to 10, distinguished entirely by (rather minor) features of the shell. In his studies of parthenogenic reproduction, S. G. Johnson (1992, 2000; Johnson and Leefe, 1999) applied the nomina decisum, limum, geniculum, parthenum and floridense to various populations of Campeloma sampled throughout the southeast, but has personally communicated that the shell traits by which he has made these distinction were generally weak and variable (Johnson 2006).
The molecular phylogenetic study of Stelbrink et al. (2020) returned no evidence of a relationship between the shell-based taxonomy of North American Campeloma populations and DNA sequence divergence. See our three-part series of essays on the phylogeny of the worldwide Viviparidae and the taxonomy of North American Campeloma from the links below.
The widespread occurrence of parthenogenesis voids the biological species concept and necessitates a retreat to the morphological. So since we ourselves have generally been unable to distinguish the Campeloma populations inhabiting any waters of the southeastern United States, by any consistent morphological criterion, we here refer all to the oldest available name, C. decisum decisum (Say, 1817). More heavily-shelled populations occasionally inhabiting larger rivers of the interior we refer to C. decisum crassulum Rafinesque 1819, and the more lightly-shelled, higher-spired forms to C. decisum decampi.
Synonyms include brevispirum, coarctatum, cornea, decisa, dissimilis, exilis, floridense, geniculum, gibba, heros, integra, integrum, leptum, lewsi, limosa, limum, milesi, milesii, obesum, parthenum, ponderosum, rufum, subsolida, subsolidum, subsolidus, and tannum. Alternate genera include Melantho, Paludina, and Vivipara.
> Supplementary Resources
- Campeloma distribution in Atlantic drainages (2023)
- Campeloma distribution in Georgia and the Florida panhandle (2025).
- Campeloma distribution in the Tennessee/Cumberland (2022)
- Campeloma in the drainage of The Ohio (2019)
- Viviparids of The Great Plains (2024)
- Pretty photo of living Campeloma decisum, courtesy of Chris Lukhaup.

- Campeloma decisum grazing on a rotten log at Stone Mountain, GA.

> Essays
- The North American genus Campeloma was mentioned parenthetically in my 9Mar21 review of the work by Stelbrink et al. (2020), A Gene Tree for the Worldwide Viviparidae.
- I reviewed the taxonomic history of Campeloma in my essay of 5Apr21, Bill and Ruth and Jack and Virginia, and Campeloma. That post features a couple nice anatomical diagrams from the work of Vail (1977).
- See my essay of 7May21 (Fun With Campeloma!) for a synthesis of evidence that all nominal species be united under the oldest available name, Campeloma decisum (Say 1817). The post includes a pdf download of the Burch/Vail dichotomous key, a review of the work of Steven Johnson, distributional maps, a phylogenetic analysis, and a nice photo of topotypic shells from Philadelphia.
> References
Burch, J. B. (1989) North American Freshwater Snails. Malacological Publications, Hamburg, Michigan. 365 pp.
Brown, K.M. & Richardson, T. D. (1992) Phenotypic plasticity in the life histories and production of two warm-temperature viviparid prosobranchs. Veliger 35: 1-11.
Brown, K. M., Varza, D.& Richardson, T. D. (1989) Life histories and population dynamics of two subtropical snails (Prosobranchia:Viviparidae). J. N. Am. Benthol. Soc. 8: 222-228.
Chamberlain, N. A. (1958) Life history studies of Campeloma decisum. The Nautilus 72: 22 - 29.
Clarke, A. (1981) The Freshwater Mollusks of Canada. The National Museums of Canada, Ottawa. 446 pp.
Clench, W. (1962) A catalogue of the Viviparidae of North America with notes on the distribution of Viviparus georgianus, Lea. Occas. Pprs. on Mollusks, Mus. Comp. Zool. Harvard, 2, 261-87
Clench, W. & Fuller, S. (1965) The genus Viviparus in North America. Occas. Pprs. on Mollusks, Mus. Comp. Zool. Harvard, 2, 385-412.
Crummett, L.T., B.F. Sears, D.C. Lafon, and M.L. Wayne (2013) Parthenogenetic populations of the freshwater snail Campeloma limum occupy habitats with fewer environmental stressors than their sexual counterparts. Freshwater Biology 58(4):655-663.
Crummett, L. T. & M. L. Wayne (2009) Comparing fecundity in parthenogenetic versus sexual populatons of the freshwater snail Campeloma limum: is there a two-fold cost of sex? Invert. Biol. 128: 1 - 8.
Dillon, R. T., Jr. (2000) The Ecology of Freshwater Molluscs. Cambridge University Press, Cambridge, England. 509 pp.
Haggerty, T.M., J.T. Garner, and L. Gilbert (2014) Density, demography, and microhabitat of Campeloma decampi (Gastropoda: Viviparidae). Walkerana 17(1):1-7.
Harvey, M., Vincent, B., & Vaillancourt, G. (1983) (Development and fecundity of Campeloma decisum (Say) (Gastropoda: Prosobranchia) in a cold climate.) Naturaliste Canadien 110: 335-342.
Imlay, M. J., Arthur, J.W., Halligan, B.J., & Steinmetz, J.H. (1981) Life Cycle of the Freshwater Snail Campeloma decisum (Viviparidae) in the Laboratory. Nautilus 95: 84-88.
Johnson, S. G. (1992) Spontaneous and hybrid origins of parthenogenesis in Campeloma decisum (freshwater prosobranch snail). Heredity 68: 253-261.
Johnson, S. G. (2000) Population structure, parasitism, and survivorship of sexual and autodiploid parthenogenetic Campeloma limum. Evolution 54: 167-175.
Johnson, S. G. (2006) Geographic ranges, populaton structure, and ages of sexual and parthenogenetic snail lineages. Evolution 60: 1417-1426.
Johnson, S. G. & Bragg, E (1999) Clonal diversity and polyphyletic origins of hybrid and spontaneous parthenogenetic Campeloma (Gastropoda: Viviparidae) from the southeastern United States. Evolution 53: 1769-1781.
Johnson, S. G. & Leefe, W. R. (1999) Evolution and ecological correlates of uniparental reproduction in freshwater snails. J. Evol. Biol. 12: 1056-1068.
Johnson, S. G., Lively, C. M. & Schrag, S. J. (1995) Age and polyphyletic origins of hybrid and spontaneous parthenogenetic Campeloma (Gastropoda: Viviparidae) from the southeastern United States. Experientia (Basel) 51: 498-509.
Johnson, S. G., & Howard, R. S. (2007) Constrasting patterns of synonymous and nonsynonymous sequence evolution in asexual and sexual frshwater snail lineages. Evolution 61: 2728-2735.
Jokinen, E. (1983) The freshwater snails of Connecticut. State Department of Environmental Protection Bulletin 109, 83 pp.
Jokinen, E. (1987) Structure of freshwater snail communities: Species-area relationships and incidence categories. Amer. Malac. Bull. 5: 9 - 19.
Karlin, A.A., Vail, V.A. & Heard, W.H. (1980) Parthenogenesis and biochemical variation in southeastern Campeloma geniculum (Gastropoda: Viviparidae). Malacol. Rev., 13: 7-15.
Richardson, T.D. & Brown, K.M. (1989) Secondary production of two subtropical snails (Prosobranchia:Viviparidae). J. N. Am. Benthol. Soc. 8: 229-236.
Selander, R.K., E.D. Parker & R.A. Browne (1977) Clonal variation in the parthenogenetic snail Campeloma decisa (Viviparidae) Veliger 20: 349-351.
Stelbrink, B., R. Richter, F. K hler, F. Riedel, E. Strong, B. Van Bocxlaer, C. Albrecht, T. Hauffe, T. Page, D. Aldridge, A. Bogan, L-N. Du, M. Manuel-Santos, R. Marwoto, A Shirokaya, and T. Von Rintelen (2020) Global diversification dynamics since the Jurassic: Low dispersal and habitat-dependent evolution explain hotspots of diversity and shell disparity in river snails (Viviparidae). Systematic Biology 69: 944 961.
Thompson, F. G. (1999) An Identification Manual for the Freshwater Snails of Florida. Walkerana 10 (23): 1 96.
Vail, V.A. (1977) Comparative reproductive anatomy of 3 viviparid gastropods. Malacologia 16: 519-520.
Vail, V.A. (1978) Seasonal reproductive patterns in 3 viviparid gastropods. Malacologia 17: 73-97.