
North Carolina spans four ecoregions (at US EPA Level III): Blue Ridge, Piedmont, the Southeastern Plains and the Middle Atlantic Coastal Plain. These areas drain toward the Atlantic through six major river systems: the Dan/Roanoke, the Tar, the Neuse, the Cape Fear, the Yadkin/Pee Dee, and the Catawba, as well as several smaller systems. The Blue Ridge ecoregion also extends across the eastern continental divide to include one tributary of the Ohio River and several tributaries of the Tennessee River not covered here (but see below).
Charlotte Dawley (1965) published a simple checklist of the freshwater gastropods of North Carolina based on collections then housed at the Academy of Natural Sciences and the University of Michigan, together with her personal observations and those of Walter (1956). From NC Atlantic drainages she documented the 22 species, with 8 synonyms. From a modern systematic standpoint, however, the freshwater gastropods of North Carolina were essentially unsurveyed prior to the present report.
Nevertheless, the state has seen considerable primary research conducted on its freshwater gastropod populations. North Carolina populations of the pleurocerid genus Pleurocera (formerly Goniobasis) have served as important models in evolutionary biology (Dillon 1984, 1988, Dillon & Reed 2002, Dillon & Frankis 2004, Stiven & Kreiser 1994), as well as occasional studies of a more ecological nature (Foin & Stiven 1970, Dillon & Davis 1991). North Carolina populations of Campeloma have figured in research on the origin of parthenogenesis (Johnson 1992). A great many important studies on the relationships between pulmonate snails and their trematode parasites have been conducted in Charlie’s Pond, north of Winston-Salem (e.g., Snyder & Esch 1993, Sapp & Esch 1994, Esch et al. 1997), and some parasitological interest has been directed toward NC populations of Pleurocera (or Goniobasis) as well (Barger & Esch 2000, Lang 1968). A unique species of caddisfly has recently been described from the Little River of Montgomery County, with a larval stage predatory on Somatogyrus virginicus (Morse & Lenat 2005). The ecological experiments of McCollum et al. (1998), although conducted in aquaria, were designed to mimic natural communities of fish, snails, and periphyton typical of the Raleigh-Durham area.
The present survey focuses on the gastropod fauna of the Atlantic drainages only. The gastropod fauna of the Tennessee River tributaries draining the 15 westernmost counties of North Carolina is covered by the FWGTN survey elsewhere on this site. Our long term plans include an extension of coverage to include upper New River tributaries in the extreme northwestern corner of the state, as resources permit.
> Methods
The database here analyzed includes 3,809 records (9Oct23) from three primary sources. The largest fraction (1,824 records) are from field observations collected by contractors and staff of the NC Wildlife Resources Commission 1977–2002 and tabulated by BTW. Approximately 363 records are from the catalogued collections of the North Carolina Museum of Natural Sciences in Raleigh, 1958–2004. An additional sample of approximately 1,428 records are from macrobenthic collections made by the NC Division of Water Quality 1985–2005, housed at the NCSM but not catalogued as of 2005. The remainder of the records were from personal collections, national museums, and miscellaneous.
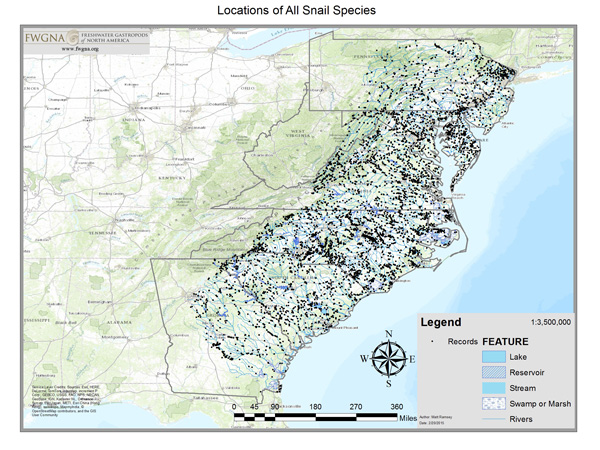 |
As the FWGNA project has expanded, we have been accorded the opportunity to examine the systematic collections of seven other national or regional museums, in addition to the NCSM: the National Museum of Natural History (Smithsonian), the Academy of Natural Sciences of Philadelphia, The Carnegie Museum of Natural History (Pittsburgh), the Delaware Museum of Natural History, the Virginia Museum of Natural History (Martinsville), the Georgia Museum of Natural History (Athens), and the Florida Museum of Natural History (Gainesville). Although the North Carolina holdings of some of these other institutions have not been extensive, their curatorial staffs have always been most helpful.
Ultimately our sample sites were located throughout the Atlantic drainages of North Carolina, in all ecoregions, all subdrainages, and all counties. Click the image above to download a pdf map. No “absence stations” are shown. If freshwater gastropods were not collected at a site, then no record resulted.
Collecting methods have varied greatly. The NCDWQ samples are semi-quantitative, taken by EPA standard methods (Barbour et al. 1999) combining kick-nets, timed searches, etc. Other collections were entirely qualitative, the result of simple untimed searches (Dillon 2006). All collections are now or soon will be housed at the NCSM, or (in the case of NCWRC) are extensively vouchered there. Our entire 3,809 record database is available (as an excel spreadsheet) from the senior author upon request.
The taxonomy employed by the FWGNA project is painstakingly researched, well-reasoned and insightful. Needless to say, it often differs strikingly from the gastropod taxonomy in common currency among casual users and most natural resource agencies. First-time visitors looking for information about particular species or genera might profitably begin their searches with a check for synonyms in our alphabetical index.
> Results
The 38 species and subspecies of freshwater gastropods we have confirmed from North Carolina (plus 2 unconfirmed) are figured in the FWGNC gallery and distinguished on the FWGNC dichotomous key. Ecological and systematic notes for each species and subspecies are provided on dedicated pages, together with regional distribution maps. Their distributions and abundances on a continental scale are analyzed in our sections on Biogeography and Synthesis.
> Acknowledgements
We thank Dr. Art Bogan and Ms. Jamie Smith for hosting us graciously at the North Carolina State Museum, providing technical assistance in a most timely and efficient manner. Gracious hosts at the other museums we have visited over the course of this project have included Bob Hershler at the USNM, Gary Rosenberg, Paul Callomon and Amanda Lawless at the ANSP, Tim Pearce at the CMNH, Liz Shea at the DMNH, Richard Hoffman at the VMNH, Liz McGhee at the GMNH, and John Slapcinsky at the FMNH.
The success of this project has in large part depended on the GIS and data analysis skills of Dr. Doug Florian and the great patience of web wizard Steve Bleezarde. Funding was provided by a subcontract from Normandeau Associates to compile a habitat fragmentation study on behalf of Yadkin Inc. Web development was supported by a Board of Directors grant from the Sierra Club.
> References
Barbour, M., J. Gerritsen, B.
Snyder, & J. Stribling (1999) Rapid bioassessment
protocols for use in streams and wadeable rivers: Periphyton, benthic
macroinvertebrates, and fish, Second edition. Washington, DC, US EPA
841-B-99-002.
Barger, M., & G.
Esch (2000) Plagioporos
sinitsini (Digenea: Opecoelidae): A one-host life cycle.
J. Parasit., 86: 150-153.
Dawley, C. (1965)
Checklist of freshwater mollusks of North Carolina. Sterkiana
19: 35-39.
Dillon, R. T., Jr. (1984)
Geographic distance, environmental difference, and divergence between
isolated populations. Syst. Zool. 33: 69-82.
Dillon, R. T., Jr. (1988)
Minor human disturbance influences biochemical variation in a populaton
of freshwater snails. Biol. Conserv., 43: 137-144.
Dillon, R.T., Jr. (2006)
Freshwater Gastropoda. pp 251 - 259 In
The Mollusks, A Guide to their Study, Collection, and Preservation.
Sturm, Pearce, & Valdes (eds.) American Malacological Society,
Los Angeles & Pittsburgh.
Dillon, R. T., Jr.,
& E. Benfield (1982) Distribution of pulmonate
snails in the New River of Virginia and North Carolina U.S.A.:
Interaction between alkalinity and stream drainage area. Freshwater
Biology 12: 179-186.
Dillon, R. T., Jr.,
& K. Davis (1991) The diatoms ingested by
freshwater snails: temporal, spatial, and interspecific variation.
Hydrobiologia 210: 233-242.
Dillon, R. T., Jr.
& R. Frankis (2004) High levels of mitochondrial
DNA sequence divergence in isolated populations of the freshwater snail
genus Goniobasis.
Amer. Malac. Bull. 19: 69-77.
Dillon, R. T., Jr., & A. J. Reed (2002) A survey
of genetic variation at allozyme loci among Goniobasis
populations inhabiting Atlantic drainages of the Carolinas. Malacologia
44: 23-31.
Esch, G., E. Wetzel, D.
Zelmer, & A. Schotthoefer (1997) Long-term changes
in parasite population and community structure: A case history. Amer.
Midl. Natur. 137: 369-387.
Flowers, J., & C.
Grover (1993) New molluscan (Gastropoda and Bivalvia)
records for the Neuse River basin, North Carolina. Brimleyana 19:
61-64.
Foin, T., & A.
Stiven (1970) The relationship of environment size and
population parameters in Oxytrema
proxima (Say) (Gastropoda: Pleuroceridae). Oecologia
(Berl.) 5: 74-84.
Johnson, S. (1992)
Spontaneous and hybrid origins of parthenogenesis in Campeloma decisum
(freshwater prosobranch snail). Heredity 68: 253-261.
Lang, B. (1968)
Note on ecology of Goniobasis
proxima in North Carolina. Nautilus 82: 3-5.
McCollum, E., L. Crowder,
& S. McCollum (1998) Complex interactions of fish,
snails, and littoral zone periphyton. Ecology, 79: 1980-1994.
Morse, J., & D.
Lenat (2005) A new species of Ceradea
(Trichoptera: Leptoceridae) preying on snails. J. N. Am. Benth. Soc.
24: 872-879.
Sapp, K., & G.
Esch (1994) The effects of spatial and temporal
heterogeneity as structuring forces for parasite communities in Helisoma anceps and
Physa gyrina.
Amer. Midl. Natur. 132: 91-103.
Snyder, S., & G.
Esch (1993) Trematode community structure in the pulmonate
snail Physa gyrina.
J. Parasit. 79: 205-215.
Walter, W. M. (1956)
Mollusks of the upper Neuse River basin, North Carolina. J.
Elisha Mitchell Soc. 72: 262 - 274.
